|
Case Report
Idiopathic dendriform pulmonary ossification incidentally found in a patient with gastroesophageal reflux disease: Case report and literature review
1 Histopathology Consultant, Pathology Department, Salmaniya Medical Complex, Manama, Kingdom of Bahrain
2 Pathology Resident, Ministry of Health, Saudi Arabia
3 Pathology Resident, King Fahad University Hospital, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
4 Radiology Specialist, Salmaniya Medical Complex, Kingdom of Bahrain
Address correspondence to:
Safa Alshaikh
Histopathology Consultant, Pathology Department, Salmaniya Medical Complex, Manama,
Kingdom of Bahrain
Message to Corresponding Author
Article ID: 100111Z06SA2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Alshaikh S, Alkhunaizy Z, Alabdullattif H, Alkhaja M. Idiopathic dendriform pulmonary ossification incidentally found in a patient with gastroesophageal reflux disease: Case report and literature review. Case Rep Int 2022;11(2):16–19.ABSTRACT
Introduction: Diffuse pulmonary ossification was firstly described by Luschka in 1856. It has two distinct patterns with different clinical, radiological, and histological associations: nodular pulmonary ossification (NPO) and dendriform pulmonary ossification (DPO). Dendriform pulmonary ossification is described by the presence of branching metaplastic osseous spicules usually with bone marrow elements. Dendriform pulmonary ossification is described by the presence of branching metaplastic osseous spicules usually with bone marrow elements. Dendriform pulmonary ossification can occur as idiopathic or in association with chronic lung diseases. Idiopathic DPO is a rare entity and has association with chronic gastric acidity.
Case Report: A 36-year-old gentleman, unknown to have any medical illness, was incidentally found to have bilateral diffuse high-density lung opacities during workup for back pain. The patient was asymptomatic. High resolution computed tomography (HRCT) of the chest showed bilateral ossified tiny branching opacities mainly involving the lower lobes. Histologically, the sections showed lung parenchyma with mature bone formation in the interstitial pulmonary spaces with fatty marrow in some of the bony spicules. Complementary upper gastrointestinal study was conducted confirming the presence of high-volume gastro-esophageal reflux.
Conclusion: Dendriform pulmonary ossification is a rare entity that can cause serious complications such as respiratory failure. Thus, awareness of it as a differential diagnosis of chronic lung disease is necessary. More studies are needed to establish treatment guidelines and long-term prognosis.
Keywords: Dendriform pulmonary ossification, Hematopoietic cells, Nodular pulmonary ossification
INTRODUCTION
Diffuse pulmonary ossification is an uncommon slowly progressive chronic disease which is characterized by the presence of widespread metaplastic bone within the lung. It has two distinct patterns with characteristic clinical, radiological, and histological association: nodular pulmonary ossification (NPO) and dendriform pulmonary ossification (DPO) [1],[2],[3],[4]. Nodular pulmonary ossification is more common and usually associated with underlying cardiac disorders. It is defined by the presence of rounded metaplastic ossifying masses in the alveolar spaces. In contrast, DPO is rarer and is described by the occurrence of dendriform, also referred to as “branching-like” spicules of metaplastic bone frequently found with bone marrow elements [5],[6],[7],[8]. It is commonly seen in association with chronic lung diseases. Nevertheless, idiopathic DPO cases have been reported. Recent studies showed that idiopathic DPO appeared to be associated with gastroesophageal reflux disease (GERD) [5]. Carney et al. stated that less than 10 cases of idiopathic DPO cases had been discussed in the literature [4]. Herein, we report a case of idiopathic DPO in a 36-year-old asymptomatic male who was incidentally found to have bilateral diffuse lung opacities on plain radiograph during his workup for back pain.
CASE REPORT
A 36-year-old gentleman, unknown to have any medical illness, was incidentally found to have bilateral generalized high-density lung opacities during plain radiograph workup for back pain. The patient had no pulmonary related symptoms. His physical examination was unremarkable.
High resolution computed tomography (CT) of the chest showed bilateral ossified tiny branching opacities mainly involving the lower lobes at the sub-pleural, septal, and peri-fissural regions. However, no honeycombing, fibrotic changes or traction were observed.
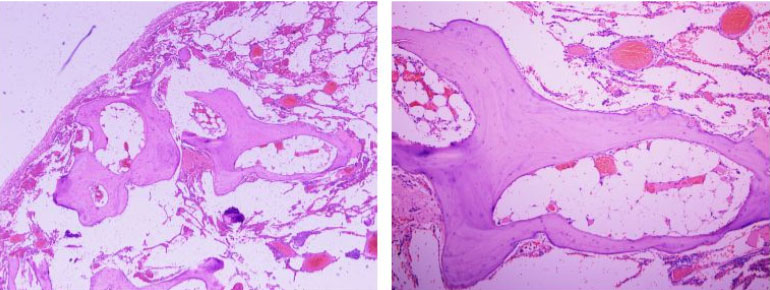
Then, the patient underwent right thoracoscopic lung biopsy under general anesthesia. Macroscopically, the lung biopsy consisted of two pieces of tan gray tissue, the larger measuring 3.0×1.0×0.4 cm, cut section revealed calcified areas. Histologically, the hematoxylin and eosin (H&E) sections showed lung parenchyma with mature bone formation in the interstitial pulmonary spaces with fatty marrow in some of these bony spicules (Figure 1).
Complementary upper gastrointestinal study was performed confirming the presence of moderate to severe gastro-esophageal reflux disease (GERD). According to the pathological features and CT scan findings, a diagnosis of DPO was established.
Follow-up
The patient’s medical records were reviewed. After surgical intervention and the diagnosis of diffuse pulmonary ossification was made, this patient traveled abroad for seeking treatment and no follow-up at our hospital.
DISCUSSION
Pulmonary metaplastic ossification can be seen as localized or diffuse form, primary or secondary to various diseases. The localized form is mostly found incidentally with dystrophic calcification in many conditions such as abscess, tuberculous scar, or nonspecific areas of lung fibrosis [1],[2],[6].
Diffuse pulmonary ossification is a rare slowly progressive chronic process, firstly described by Luschka in 1856 [9] and is defined by the presence of extensive metaplastic bone within the pulmonary interstitium and alveolar walls [9]. There are two main patterns of diffuse pulmonary ossification with different clinical, radiological, and histological associations: NPO and DPO.
Nodular pulmonary ossification was firstly described by Salivger et al. in association with mitral valve stenosis in 1933 [10]. Since then, it has been documented in patients with pulmonary congestion in the context of cardiac diseases such as left ventricular dysfunction, atrial fibrillation, and post-myocardial infarction [4],[11]. Radiologically, it is recognized as small round nodules. Histological findings include rounded masses of mature bone in the alveolar spaces without any bone marrow elements [6]. The hypothesized pathophysiology behind NPO involves osseous fibroblast metaplasia induced by hemosiderin deposition in chronic venous congestion situations or organization of alveolar exudates [12],[13].
In contrast to NPO, DPO is described histologically by formation of branching—“racemouce” or “reticular”—metaplastic osseous bony spicules with usually bone marrow elements (fatty or hematopoietic cells) in the pulmonary interstitium or alveolar walls [4]. In imaging studies, manifests as tree-like “linear” opacities with predilection to lower lung and is predominantly found in older men in their fifth to sixth decade of life with a male to female ratio 6:1 [5],[8],[14]. Patients with DPO are commonly asymptomatic; therefore, most of the early reported cases in the literature were diagnosed postmortem with occurrence ratio of 1.63 cases DPO/1000 autopsies [15]. Furthermore, DPO can occur as idiopathic or in association with chronic lung diseases. The latter association was reported as idiopathic pulmonary fibrosis, usual interstitial pneumonia, adult respiratory distress syndrome, organizing pneumonia, asbestosis, heavy metals exposure, pneumothorax, and osteogenesis imperfecta [5],[15],[16],[17]. Additionally, the coexistence of DPO with pulmonary and extra-pulmonary malignancies has been reported as lung adenocarcinoma, metastatic melanoma [18], mucoepidermoid carcinoma [19], and duodenal adenocarcinoma [20].
Idiopathic DPO is a rare entity in which only osseous branching bony spicules are found within the lung parenchyma without any other histological findings. It has been theorized that the presence of acidic medium and hypoxia can cause osseous fibroblastic metaplasia [5]. Gruden et al. described that DPO without usual interstitial pneumonia seems to be associated with GERD [5]. Similarly, Fernandez-Bussy et al. identified a case of DPO in a 43-year-old man with history of GERD [15].
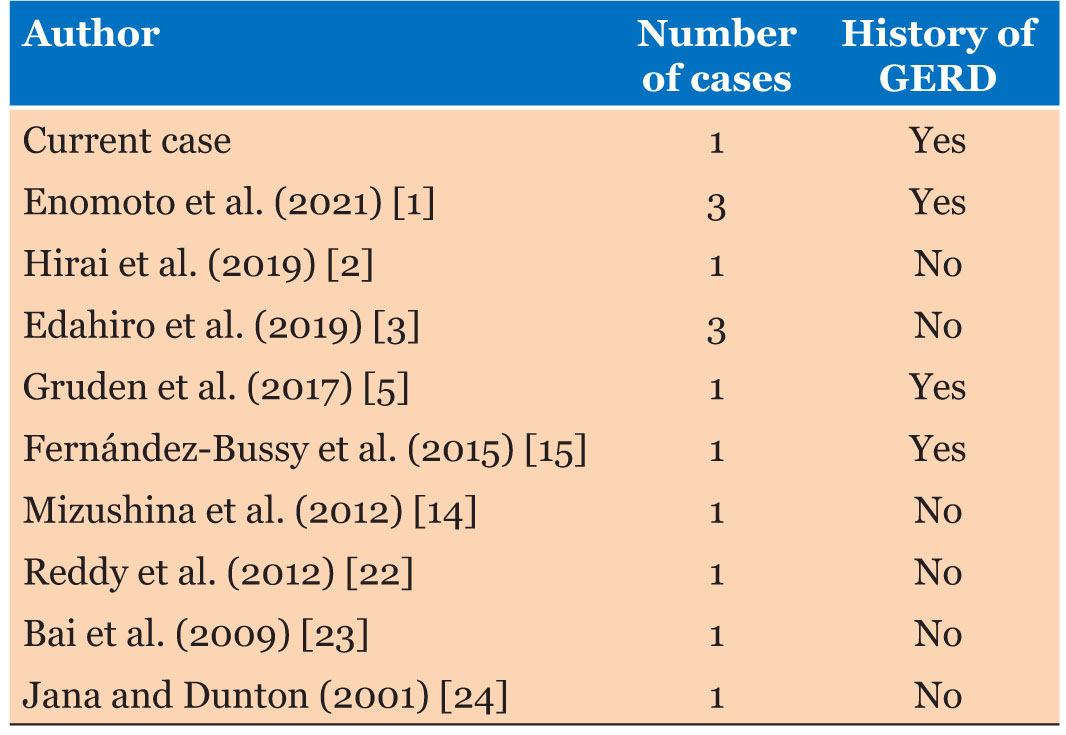
Carney et al. stated that less than 10 cases of idiopathic DPO were documented in the English literature [4]. However, we found less than 15 cases of idiopathic DPO in which there were no other clinical, radiological, or histological findings (Table 1).
Definitive antemortem diagnosis of DPO requires lung biopsy by Video Assisted Thoracoscopy (VATS), transbronchial, or by thoracotomy in concurrence with chest computed tomography (CT) appearance [6].
Diffuse pulmonary ossification can produce serious complications such as respiratory failure ending up with lung transplantation. Henceforth, awareness of this entity as a differential diagnosis of chronic lung diseases is necessary because it is, usually, misinterpreted radiologically as pulmonary fibrosis, bronchiectasis, or lymphangitic spread of tumor [4],[19].
No effective management or treatment guidelines of DPO are published. Trails of low calcium diet, calcium-binding drugs, bisphosphates, and warfarin are not shown to be effective [6],[11]. Tomoko et al. suggested possible remission of DPO with corticosteroids [21].
More studies related to pathophysiology, risk factors, and treatment options are required to establish definitive management guidelines and to determine the long-term prognosis.
CONCLUSION
Dendriform pulmonary ossification is a rare entity that can lead to serious complications such as respiratory failure. Therefore, clinicians, radiologists, and pathologists should be aware of as a differential diagnosis of chronic lung diseases. More studies are needed to establish treatment guidelines and long-term prognosis.
REFERENCE
1.
Enomoto T, Takimoto T, Kagawa T, et al. Histologically proven dendriform pulmonary ossification: A five-case series. Intern Med 2021;60(14):2261–8. [CrossRef]
[Pubmed]

2.
Hirai S, Katayama T, Inoue S. Idiopathic diffuse pulmonary ossification diagnosed by lung biopsy. [Article in Japanese]. Kyobu Geka 2019;72(5):360–2.
[Pubmed]

3.
Edahiro R, Kurebe H, Nakatsubo S, et al. Three cases of idiopathic diffuse pulmonary ossification. Intern Med 2019;58(4):545–51. [CrossRef]
[Pubmed]

4.
Carney JM, Mammarappallil JG, Sporn TA, Pavlisko EN. Dendriform pulmonary ossification leading to bilateral lung transplant: A case report. Virchows Arch 2018;473(3):379–83. [CrossRef]
[Pubmed]

5.
Gruden JF, Green DB, Legasto AC, Jensen EA, Panse PM. Dendriform pulmonary ossification in the absence of usual interstitial pneumonia: CT features and possible association with recurrent acid aspiration. AJR Am J Roentgenol 2017;209(6):1209–15. [CrossRef]
[Pubmed]

6.
Kim DU, Guinee D, Mohammed TLH. Case of the season: Usual interstitial pneumonia with dendriform pulmonary ossification. Semin Roentgenol 2015;50(1):4–7. [CrossRef]
[Pubmed]

7.
Harvey NT, Heraganhally S, Au V, Ellis D, Klebe S, Henderson DW. Idiopathic diffuse dendriform pulmonary ossification in a dental technician. Pathology 2012;44(4):363–5. [CrossRef]
[Pubmed]

8.
Bisceglia M, Chiaramonte A, Panniello G, Tucci A, Orcioni GF, Colby TV. Selected case from the Arkadi M. Rywlin international pathology slide series: Diffuse dendriform pulmonary ossification: Report of 2 cases with review of the literature. Adv Anat Pathol 2015;22(1):59–68.
[Pubmed]

9.
Felson B, Schwarz J, Lukin RR, Hawkins HH. Idiopathic pulmonary ossification. Radiology 1984;153(2):303–10. [CrossRef]
[Pubmed]

10.
Galloway RW, Epstein EJ, Coulshed N. Pulmonary ossfic nodules in mitral value disease. Br Heart J 1961;23(3):297–307. [CrossRef]
[Pubmed]

11.
Morikawa M, Fukuda Y, Terasaki Y, et al. Osteogenesis imperfecta associated with dendriform pulmonary ossification. Am J Respir Crit Care Med 2016;193(4):460–1. [CrossRef]
[Pubmed]

12.
Peros-GolubicićT, Tekavec-Trkanjec J. Diffuse pulmonary ossification: An unusual interstitial lung disease. Curr Opin Pulm Med 2008;14(5):488–92. [CrossRef]
[Pubmed]

13.
Popelka CG, Kleinerman J. Diffuse pulmonary ossification. Arch Intern Med 1977;137(4):523–5.
[Pubmed]

14.
Mizushina Y, Bando M, Hosono T, et al. A rare case of asymptomatic diffuse pulmonary ossification detected during a routine health examination. Intern Med 2012;51(20):2923–7. [CrossRef]
[Pubmed]

15.
Fernández-Bussy S, Labarca G, Pires Y, Díaz JC, Caviedes I. Dendriform pulmonary ossification. Respir Care 2015;60(4):e64–7. [CrossRef]
[Pubmed]

16.
Yoon HK, Moon HS, Park SH, Song JS, Lim Y, Kohyama N. Dendriform pulmonary ossification in patient with rare earth pneumoconiosis. Thorax 2005;60(8):701–3. [CrossRef]
[Pubmed]

17.
Abe J, Oura H, Niikawa H, Yaegashi H, Kondo T. Dendriform pulmonary ossification: Unusual cause of spontaneous pneumothorax. Thorax 2014;69(1):97–8. [CrossRef]
[Pubmed]

18.
Kayser K, Stute H, Tuengerthal S. Diffuse pulmonary ossification associated with metastatic melanoma of the lung. Respiration 1987;52(3):221–7. [CrossRef]
[Pubmed]

19.
Triki M, Kallel R, Hentati A, Hentati Y, Mnif H, Boudawara T. Dendriform pulmonary ossification in a patient with mucoepidermoid carcinoma. Asian Cardiovasc Thorac Ann 2016;24(6):604–6. [CrossRef]
[Pubmed]

20.
Ohtsuki Y, Mori K, Ohnishi H, et al. Investigation of aluminum and iron deposition on metaplastic bones in three patients with diffuse pulmonary ossification. Med Mol Morphol 2015;48(4):235–8. [CrossRef]
[Pubmed]

21.
Yamagishi T, Fujimoto N, Miyamoto Y, et al. The rapid appearance and disappearance of dendriform pulmonary ossification after diffuse alveolar hemorrhage. Am J Respir Crit Care Med 2016;193(3):333–4. [CrossRef]
[Pubmed]

22.
Reddy TL, von der Thüsen J, Walsh SLF. Idiopathic dendriform pulmonary ossification. J Thorac Imaging 2012;27(5):W108–10. [CrossRef]
[Pubmed]

23.
Bai P, Sun YC, Chen DN, Jin JM, Zhuo J, Liu HG. Idiopathic diffuse pulmonary ossification: A case report and review of the literature. [Article in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi 2009;32(8):588–92.
[Pubmed]

24.
Jaderborg JM, Dunton RF. Rare clinical diagnosis of dendriform pulmonary ossification. Ann Thorac Surg 2001;71(6):2009–11. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Safa Alshaikh - Conception of the work, Design of the work, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Zahra Alkhunaizy - Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hanoof Alabdullattif - Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Maryam Alkhaja - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Safa Alshaikh et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.