|
Case Report
Aortoesophageal fistula: A case report
1 Hospital Espírito Santo de Évora, Largo do Sr. da Pobreza, 7000-811 Évora, Portugal
2 Hospital de Santa Marta, R. de Santa Marta 50, 1169-024 Lisboa, Portugal
Address correspondence to:
Miguel Rocha Melo
Largo Senhor da Pobreza, 7000-811 Évora,
Portugal
Message to Corresponding Author
Article ID: 100109Z06MM2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Melo MR, Bento R, Oliva A, Ribeiro S, Félix R, Carvalho M. Aortoesophageal fistula: A case report. Case Rep Int 2022;11(2):5–10.ABSTRACT
Introduction: Aortoesophageal fistula (AEF) is a rare and potentially lethal cause of upper gastrointestinal bleeding. Although several causes have been implicated in this condition, a ruptured thoracic aortic aneurysm remains the most common cause. Despite the challenge of differential diagnosis, this is a situation that requires prompt action with immediate hemorrhage control.
Case Report: We report a case of a 56-year-old woman who presented with hemorrhagic shock secondary to AEF with no underlying cause identified, treated with Thoracic Endovascular Aortic Repair (TEVAR) after immediate bleeding control with a Sengstaken–Blakemore (SB) tube. Post-operatively the patient developed pneumonia and operative wound infection that were treated conservatively. At 24 months follow-up, the patient is asymptomatic and there is no clinical, analytical, or imagiological evidence of vascular graft infection.
Conclusion: Survival outcomes have improved with the advent of endoluminal aortic stent therapies and TEVAR is currently considered a viable approach for AEF bleeding control in the emergency setting for unstable patients. However, long-term concerns remain since TEVAR is not a definitive treatment for AEF defect, leaving the patients at risk for AEF recurrence but also for stent graft infection. Resection of the esophagus with restoration of gastrointestinal continuity and vascular reconstruction can be performed in a one or two stage procedure and is the definitive treatment for AEF.
Keywords: Aortoesophageal fistula, Emergency bleeding control, Gastrointestinal bleeding, Hemorrhagic shock
INTRODUCTION
Aortoesophageal fistula (AEF) is a rare but potentially lethal cause of upper gastrointestinal bleeding. Most of the cases present with life-threatening massive bleeding and the majority of these patients die before a definitive diagnosis can be made [1],[2].
The main cause is related to rupture of descending thoracic aorta aneurysm into the esophagus [3],[4]. Other causes include foreign body ingestion, esophageal cancer, and previous aortic surgery [5],[6],[7],[8].
Patients with acute bleeding due to an AEF can be treated successfully in the emergency setting by TEVAR. However, long-term results remain unclear as this technique is associated with various severe complications, such as vascular graft infection and development of new fistulous tract [9],[10],[11].
According to the most recent guidelines, a definitive surgical procedure should take place as soon as the patient is stabilized for such a complex procedure [12].
We report a case of a patient who presented with hemorrhagic shock secondary to primary AEF, diagnosed with intraoperative esophagogastroduodenoscopy (EGD) and confirmed with computed tomography (CT) angiogram after bleeding control was achieved with a Sengstaken–Blakemore (SB) tube. The patient was treated with TEVAR and remains a candidate for a definitive surgical approach.
CASE REPORT
We present a case of a 56-year-old woman with comorbid condition of hypertension with complaints of sudden hematemesis. The patient was conscious and hemodynamically stable on arrival and was transferred to the hospital emergency department (ED). Upon arrival at ED, nasogastric intubation confirmed the presence of blood in the stomach. Arterial blood gas (ABG) showed mildly elevated lactate (1.7 mmol/L) and a hemoglobin level of 7.7 g/dL. The patient had another two episodes of hematemesis, and two red blood cell units (RBC) were administrated.
The patient underwent EGD that showed abundant blood clots in the stomach and no source of hemorrhage was identified. Suddenly, the patient develops hemodynamic instability with another episode of hematemesis requiring rapid fluid resuscitation, and massive blood transfusion protocol was activated.
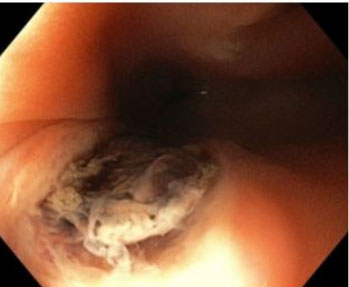
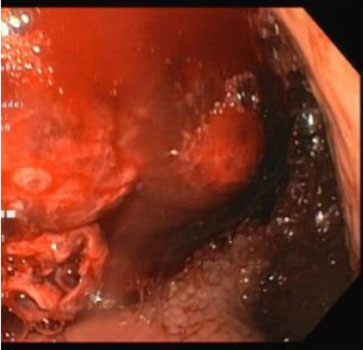
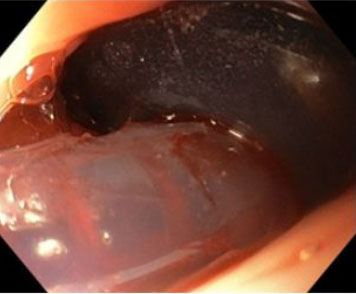
The surgical team decided for an emergency laparotomy. Given the high suspicion of gastric ulcer, an anterior gastrostomy was performed with identification of large blood clots in the stomach. At this point, a gastric ulcer was excluded and intraoperative EGD revealed an esophageal ulcer in the middle esophagus with an adherent clot (Figure 1). Clot dislodgement occurred during the procedure with massive bleeding (Figure 2) and a transgastric Foley catheter (28 Fr) was placed for immediate hemorrhage control (Figure 3). Foley catheter was then replaced by a Sengstaken–Blakemore (SB) tube and bleeding was controlled by gradually inflating the esophageal balloon up to 120 mmHg. The patient remained stable after receiving a total of 14 red blood cell units, 12 units of fresh frozen plasma, 3 units of platelets, and 10 g of fibrinogen. Both gastrostomy and laparotomy were primarily closed.
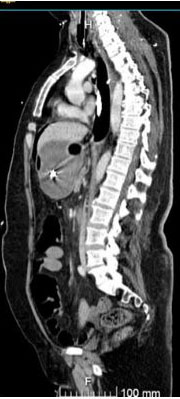
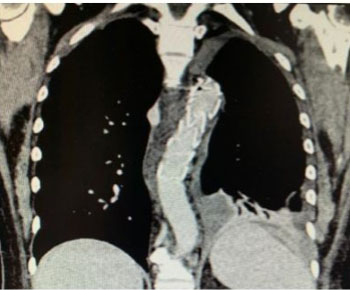
Immediately after the surgical intervention, the patient underwent computed tomography (CT) angiogram which showed contained leakage of contrast from the anterior portion of descending thoracic aorta to the esophagus confirming the diagnosis of AEF (Figure 4).
The patient remained hemodynamically stable and was transferred to another hospital with a vascular surgery unit and underwent emergent TEVAR of the descending thoracic aorta (Figure 5). Intraoperative angiogram evidenced fistula exclusion with permeability of the supra-aortic trunks.
Post-operatively, the patient was transferred to intensive care unit (ICU). Sengstaken–Blakemore tube pressure was decreased 11 hours after its placement, and it was removed two days later with no evidence of esophageal necrosis. Post-operatively the patient developed pneumonia and operative wound infection that were successfully treated with large spectrum antibiotics. The patient was extubated 17 days after the endovascular procedure and discharged nine days later under chronic prophylactic antibiotics.
At follow-up, six months later an EGD was performed, revealing prominent mucosa at 25 cm with an extension of about 8 mm in relation with scar of previous fistula.
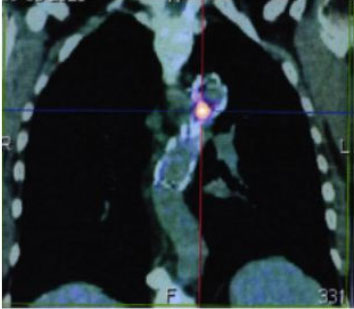
18F-Fludeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) was performed 12 months after discharge, revealing an unspecified periprosthetic inflammatory process and a small mediastinal abscess (SUVmax 11.65) between the anterior aspect of the descending thoracic aorta and the esophagus (in relationship to previous fistula level) (Figure 6).
DISCUSSION
Aortoesophageal fistula (AEF) is a rare and potentially fatal cause of massive upper gastrointestinal bleeding. The first case was described by Dubreuil in 1818 [13].
The AEF can be classified as primary or secondary based on the etiologic mechanism. Primary AEF is mainly caused by rupture of an aortic aneurysm [6]. Other causes include esophageal ulcer, malignancies [14], congenital structural anomalies [15], and vasculitis [16]. Secondary causes include foreign body ingestion, direct trauma, radiotherapy, and invasive procedural instrumentation as surgical or endovascular repair of an aortic aneurysm [6].
Chiari first described the typical clinical presentation of AEF as a triad of mid-thoracic pain or dysphagia, a small self-limiting arterial hemorrhage, followed by an interval free of symptoms and overt exsanguination after a few hours [17]. In this case, no complains of thoracic pain or dysphagia were referred by our patient. The sentinel bleeding is caused by the establishment of a fistula into the nearby esophagus, and it usually presents as small or intermittent hemorrhage due to the thrombus formed within the fistula as a result of hypotension [18]. Increase of blood pressure results in clot dislodgement with subsequent fatal exsanguination [19].
Esophagogastroduodenoscopy is useful for the diagnosis of AEF since it is widely available and has high specificity and sensibility. Additionally, EGD is a useful modality to rule out differential diagnoses leading to upper gastrointestinal bleeding [20]. In our case, the first EGD showed abundant blood clots in the stomach but no description of esophageal lesions as ulcers or varices was made. In the second EGD, an esophageal ulcer with adherent clot was detected raising suspicion of underlying vascular fistula.
Computed tomography is usually performed to confirm the diagnosis. The suggestive findings for AEF include contrast penetrating into the lumen or air within the aortic wall [21]. In this case, CT was performed after emergency surgery for bleeding control, with angiogram demonstrating the fistula contained by SB tube.
Conservative treatment of an AEF is almost invariably fatal. In patients presenting active and life-threatening bleeding from an AEF, emergency endovascular graft insertion should be proposed as the primary strategy to control bleeding and restore hemodynamic stability [12],[22]. Endovascular stent grafting is faster and safer than surgery in unstable patients, but the major concerns are the long-term results of this approach. Thoracic Endovascular Aortic Repair does nothing to address the issue of the defect in the digestive tract, leaving the patients at risk of AEF recurrence and/or stent graft infection [23],[24].
According to the current European Guidelines on the Management of Vascular Graft and Endograft Infections, patients with AEF complicated by vascular graft/endograft infection, excision of the infected material, repair of the esophagus, and coverage with viable tissue is recommended as definitive treatment. In our case, 18F-FDG PET/CT performed 12 months after discharge, showed linear uptake with projection of the vessel but not suggestive of volunteered geographic information (VGI), plus a small abscess between descending aorta and esophagus in relation with fistulous tract. Hence, our patient does not meet the criteria for the diagnosis of aortic graft infection [25].
Therefore, patients with acute bleeding due to an AEF can be treated successfully in the emergency setting by TEVAR, but once they have recovered, a permanent surgical procedure should be performed to achieve more long-lasting results. Several treatment options have been used to deal with AEF, including arterial in situ reconstruction, extra-anatomic bypass with concomitant primary esophageal repair, or esophagectomy with cervical esophagostomy and secondary restoration of gastrointestinal tract continuity.
Resection of the esophagus and restoration of gastrointestinal continuity can be performed in one or two stage procedure. The same principle applies to the vascular reconstruction and resection of the aortic graft. The choice of strategy to be followed will often depend on the urgency of the situation, the condition of the patient and the possibility of controlling the infection [12].
In our clinical case, after temporary control of active massive bleeding, the patient underwent an emergency TEVAR to sustain the hemorrhagic source more permanently. After explaining risks and benefits of surgical permanent repair alternatives, for the moment, the patient rejected definitive surgery. In a 24-month follow-up, under chronic prophylactic antibiotherapy, the patient is asymptomatic and there is no clinical, analytical, or imagiological evidence of vascular graft infection. The patient is being closely monitored by a multidisciplinary team as it is still a candidate to a definitive surgical procedure.
CONCLUSION
Survival outcomes have improved with the advent of endoluminal aortic stent therapies and Thoracic Endovascular Aortic Repair (TEVAR) is currently considered a viable approach for AEF bleeding control in the emergency setting for unstable patients. However, long-term concerns remain since TEVAR is not a definitive treatment for AEF defect, leaving the patients at risk for AEF recurrence but also for stent graft infection. Resection of the esophagus with restoration of gastrointestinal continuity and vascular reconstruction can be performed in a one or two stage procedure and is the definitive treatment for AEF.
REFERENCE
1.
Yang Y, Hu D, Peng D. Primary aortoesophageal fistula: A fatal outcome. Am J Emerg Med 2018;36(2):343.e1–3. [CrossRef]
[Pubmed]

2.
Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc 2001;53(1):6–13. [CrossRef]
[Pubmed]

3.
Voorhoeve R, Moll FL, de Letter JA, Bast TJ, Wester JP, Slee PH. Primary aortoenteric fistula: Report of eight new cases and review of the literature. Ann Vasc Surg 1996;10(1):40–8. [CrossRef]
[Pubmed]

4.
Hughes FM, Kavanagh D, Barry M, Owens A, MacErlaine DP, Malone DE. Aortoenteric fistula: A diagnostic dilemma. Abdom Imaging 2007;32(3):398–402. [CrossRef]
[Pubmed]

5.
Takeno S, Ishii H, Nanashima A, Nakamura K. Aortoesophageal fistula: Review of trends in the last decade. Surg Today 2020;50(12):1551–9. [CrossRef]
[Pubmed]

6.
Hollander JE, Quick G. Aortoesophageal fistula: A comprehensive review of the literature. Am J Med 1991;91(3):279–87. [CrossRef]
[Pubmed]

7.
Nandi P, Ong GB. Foreign body in the oesophagus: Review of 2394 cases. Br J Surg 1978;65(1):5–9. [CrossRef]
[Pubmed]

8.
el Barbary AS, Foad H, Fathi A. Oesophageal fistulae caused by swallowed foreign bodies. J Laryngol Otol 1969;83(3):251–9. [CrossRef]
[Pubmed]

9.
Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: Insight from the European registry on endovascular aortic repair complications. Circulation 2009;120(11 Suppl):S276–81. [CrossRef]
[Pubmed]

10.
Eggebrecht H, Mehta RH, Dechene A, et al. Aortoesophageal fistula after thoracic aortic stent-graft placement: A rare but catastrophic complication of a novel emerging technique. JACC Cardiovasc Interv 2009;2(6):570–6. [CrossRef]
[Pubmed]

11.
Chiesa R, Melissano G, Marone EM, Marrocco-Trischitta MM, Kahlberg A. Aorto-oesophageal and aortobronchial fistulae following thoracic endovascular aortic repair: A national survey. Eur J Vasc Endovasc Surg 2010;39(3):273–9. [CrossRef]
[Pubmed]

12.
Chakfé N, Diener H, Lejay A, et al. Editor’s choice – European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc Endovasc Surg 2020;59(3):339–84. [CrossRef]
[Pubmed]

13.
Rawala MS, Badami V, Rizvi SB, Nanjundappa A. Aortoesophageal fistula: A fatal complication of thoracic endovascular aortic stent-graft placement. Am J Case Rep 2018;19:1258–61. [CrossRef]
[Pubmed]

14.
Kawakami T, Tsushima T, Omae K, et al. Risk factors for esophageal fistula in thoracic esophageal squamous cell carcinoma invading adjacent organs treated with definitive chemoradiotherapy: A monocentric case-control study. BMC Cancer 2018;18(1):573. [CrossRef]
[Pubmed]

15.
Millar A, Rostom A, Rasuli P, Saloojee N. Upper gastrointestinal bleeding secondary to an aberrant right subclavian artery-esophageal fistula: A case report and review of the literature. Can J Gastroenterol 2007;21(6):389–92. [CrossRef]
[Pubmed]

16.
Getachew M, Seyoum N, Debebe F. Behcet’s disease with upper GI bleeding. Case Rep Emerg Med 2020;2020:2983209. [CrossRef]
[Pubmed]

17.
Hwang SH, Cho JW, Bae CH, Jang JS. Staged surgical treatment of primary aortoesophageal fistula. Korean J Thorac Cardiovasc Surg 2019;52(3):182–5. [CrossRef]
[Pubmed]

18.
Guinn GA, Garcia Rinaldi R, Whisennand HH. Surgical management of the bleeding aortopulmonary fistula. J Thorac Cardiovasc Surg 1976;71(4):554–6.
[Pubmed]

19.
Saers SJF, Scheltinga MRM. Primary aortoenteric fistula. Br J Surg 2005;92(2):143–52. [CrossRef]
[Pubmed]

20.
Martin M, Steele S, Mullenix P, Haque M, Andersen C. Endoscopic diagnosis of a clinically silent aortoesophageal fistula: Case report and review of the literature. Ann Vasc Surg 2004;18(3):352–6. [CrossRef]
[Pubmed]

21.
Song Y, Liu Q, Shen H, Jia X, Zhang H, Qiao L. Diagnosis and management of primary aortoenteric fistulas-experience learned from eighteen patients. Surgery 2008;143(1):43–50. [CrossRef]
[Pubmed]

22.
Jonker FHW, Heijmen R, Trimarchi S, Verhagen HJM, Moll FL, Muhs BE. Acute management of aortobronchial and aortoesophageal fistulas using thoracic endovascular aortic repair. J Vasc Surg 2009;50(5):999–1004. [CrossRef]
[Pubmed]

23.
Canaud L, Ozdemir BA, Bee WW, Bahia S, Holt P, Thompson M. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J Vasc Surg 2014;59(1):248–54. [CrossRef]
[Pubmed]

24.
Kahlberg A, Melissano G, Mascia D, Loschi D, Grandi A, Chiesa R. How to best treat infectious complications of open and endovascular thoracic aortic repairs. Semin Vasc Surg 2017;30(2–3):95–102. [CrossRef]
[Pubmed]

25.
Lyons OTA, Baguneid M, Barwick TD, et al. Diagnosis of aortic graft infection: A case definition by the management of aortic graft infection collaboration (MAGIC). Eur J Vasc Endovasc Surg 2016;52(6):758–63. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Miguel Rocha Melo - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rita Bento - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
André Oliva - Conception of the work, Design of the work, Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Susana Ribeiro - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rosa Félix - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Manuel Carvalho - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Miguel Rocha Melo et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.