| Table of Contents |  |
|
Case Report
| ||||||
| Human embryonic stem cell for the treatment of multiple sclerosis: A case report | ||||||
| Geeta Shroff | ||||||
|
MBBS, Founder and Director, H8, Green Park Extension, New Delhi, India. Email ID:geetashroff@hotmail.com
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article: |
| Shroff G. Human embryonic stem cell for the treatment of multiple sclerosis: A case report. Case Rep Int 2015;4:38–42. |
|
Abstract
|
|
Introduction:
Multiple sclerosis (MS) is a chronic demyelinating disease with inflammatory neurodegeneration. It is caused by the genesis of autoimmune response to self-antigens in a genetically susceptible individual. Currently, no remedy is available for treatment of MS. However, corticosteroids and selective immunomodulators are used. Human embryonic stem cells (hESC) have been investigated in animal models which showed the potency to mitigate the signs and symptoms of MS. We present a case of patient with MS treated with hESC therapy.
Case Report: A 34-year-old female with MS was referred to our facility. On presentation, the patient was unable to walk properly due to stiffness and paralysis in lower limbs and had significant weight loss in the last two years. The hESC therapy consisted of treatment phases separated by gap phases. After the hESC therapy, there was a remarkable improvement in the muscle bulk, tone and power of the patient. The patient experienced an increased energy level and power of upper limbs. She gained weight during the treatment, but there was no change in her walking status. Conclusion: We observed a significant improvement in the symptoms of MS with the hESC treatment. There was no adverse event observed during the treatment. | |
|
Keywords:
Autoimmune, Human embryonic stem cell, Inflammatory neurodegeneration, Multiple sclerosis
| |
|
Introduction
| ||||||
|
Multiple sclerosis (MS) is a chronic inflammatory neurological disorder caused by an autoimmune response against the self-antigens in a genetically susceptible individual [1]. The MS diagnosis is based on the lesions present in the central nervous system which appears in the different areas of brain [2]. The rate of growth of the disease is extremely mutable and uncertain, with an unclear etiology; presently there is no cure, and only symptomatic therapy is available. Generally, oral or intravenous corticosteroids like methylprednisolone is used at high dose in the routine therapy for acute attack which results in a faster recovery from the disability within a duration of three to five days of course [3]. But corticosteroid therapy does not have a significant impact on the long-term disability [4]. Human embryonic stem cells (hESC) may probably serve as an unlimited source of neural cells for transplantation in neurological disorders, such as MS patients. The safety and efficacy of the hESC has already been examined in the rodent animal model. The treatment with hESC showed a significant improvement in the extremity of the signs and symptoms and tissue damage in the central nervous system. The effect was mainly neuroprotective rather than the regenerative [5]. In our previous studies, hESC treatment had shown significant improvement in condition of patients suffering from cerebral palsy [6]. The present study evaluated the safety and efficacy of hESC therapy for the treatment of MS. The current study aims at finding the safety and efficacy of hESC on a pre-diagnosed MS patient. The clinical transplantation was performed under the oversight of an independent ethics committee (IEC). A written informed consent and video consent was obtained from the patient prior to the study. An in-house patented technology in a Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP) and Good Tissue Practices (GTP) certified laboratory at our facility (Patent-WO 2007/141657A PCT/1B 2007 Published 13 Dec 2007) is used for culture and maintenance of hESCs. The cell lines are free of animal product and are chromosomally stable. The process of cell culture and characterization has been elaborated previously [6]. The safety and efficacy of our cell line has been established and reported elsewhere [7]. The therapy consisted of treatment phases and gap phases (4 months) (Table 1). Each treatment phase was 8–12 weeks long wherein 0.25 ml (<4 million cells) hESCs were administered intramuscularly, twice a day to make sure that the recipient's immune system does not reject the stem cells ("prime" the body). About 1 ml hESCs (<16 million cells) were administered every 10 days intravenously (to "home in" to the required area) and 1 to 5 ml hESCs were administered every 7 days. The supplemental route allowed for the "local action" of hESCs by introducing them near the injured site. She also received hESC eye drops and nasal drops three times a day (every 45 minutes), twice a week. The supportive treatment included physiotherapy and occupational therapy. Patient was also prescribed supplements to maintain the normal profile of body electrolytes, vitamins and minerals. The radiological investigations for the patient were done before the start of the treatment and then at regular intervals. | ||||||
| ||||||
|
| ||||||
|
Case Report
| ||||||
|
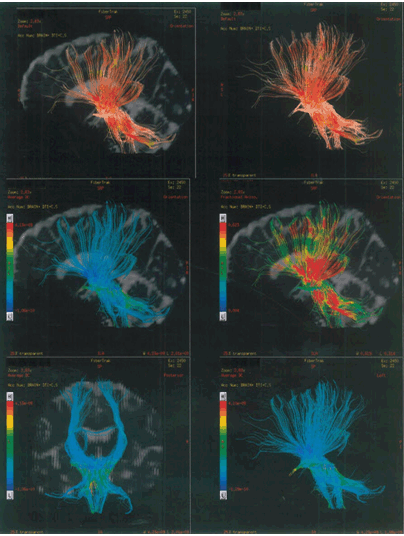
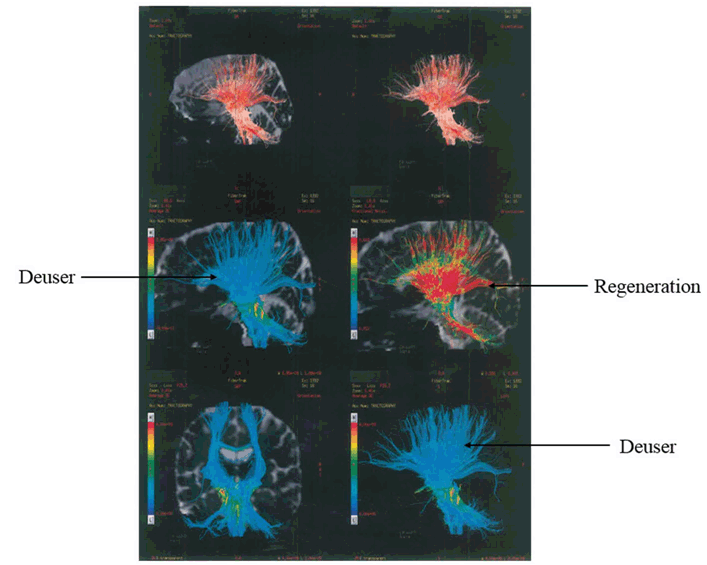
A 34-years-old female was admitted at our facility with pre diagnosed MS. The patient was healthy till March 2001, but in April 2001, she suffered with bitemporal hemianopia which was further justified by MRI scan, which revealed small lesions in the brain. In 2003, the patient suffered with urinary incontinence followed by sensory paraesthesia over right shoulder in 2005. In 2007, the sensory paraesthesia was diagnosed in the left thigh. Band like sensation over abdomen and sensory constriction and stretching feeling in hands was observed and MRI revealed lesion in spinal cord in 2008. In 2009, the patient suffered with spasticity in right leg with knee clonus and stretching sensation over left popliteal fossa. The patient was on steroid therapy and due to weakness in right leg; patient was unable to walk properly. In February 2010, stretching sensation over left popliteal fossa, right leg foot drop, problem in hip flexion and balance issues were observed. It was followed by stretching sensation over left popliteal fossa, and weakening of right leg in October 2010. It was further justified by MRI which revealed one new lesion in the brain. In January 2011, the symptoms reoccurred and the patient went on steroid therapy, which provided symptomatic relief to the patient. In April 2011, the same symptoms were experienced by the patient and the patient again underwent the steroid therapy. In October 2012, new symptoms, i.e., sensory stretching on left forearm, spasticity in left leg, heaviness while walking and burning sensation in both legs (more on left side) were observed. Magnetic resonance imaging scan showed an active and nodular lesion on right cerebrum. Patient had deteriorated gradually after year 2012, with increasing weakness in left leg. It started with increased spasticity in lower one-third of leg, followed by weakness of knee and hip. She had got multiple attacks in the year 2013 after which, she was not able to walk without support. Patient had lost weight during last two years. From April 2013 to April 2014, there was gradual deterioration in walking with left leg due to weakness. Magnetic resonance imaging scan revealed that there were lesions in the cervical cord. She was in the progressive stage of MS (Figure 1). In May 2014, the patient first visited at our facility for the treatment of MS and received the first cycle of stem cells as a part of therapy. Patient was given hESC therapy as primary treatment for two months. After two months of treatment, patient reported an improvement in muscle bulk and tone. The power of upper limbs was increased and patient had a feeling of well-being with increased energy level. Her weight also increased during the course of treatment. Patient still walks with support. The improvement in clinical signs and symptoms were evaluated by a unique numerical scoring system referred to as the Nutech Functional Scores (NFS). The evaluated parameters are given in Table 2. The details of the scoring system will be presented as a separate paper. The patient was also scored using expanded disability status scale (EDSS). The change in muscle power, bulk and tone after the hESC therapy is given in Table 3. The tractography showed that there was a mild reduction in the size of lesions in bilateral periventricular white matter and another lesion in the right occipital white matter (Figure 2). The remyelination in the spine was visible in the tractography of spine (Figure 3). | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
Discussion
| ||||||
|
Multiple sclerosis is an autoimmune disease which can cause a variety of symptoms such as hypoesthesia, muscle weakness, dysarthria or dysphagia, nystagmus, abnormal muscle spasms, or difficulty in moving, coordination and balance; optic neuritis, phosphenes or diplopia, cognitive impairment, fatigue and acute or chronic pain syndromes, bladder and bowel difficulties, or emotional symptomatology [1]. There is no study reporting the management of chronic MS with hESC therapy. The present study is the first to report the efficacy of hESC therapy in a MS patient. After the therapy, patient showed clinically significant improvement in the symptoms of the disease. After two months of treatment, the patient reported improved muscle bulk and tone; the upper limb power was increased. In addition to improvement, the patient had a feeling of well-being with an increased energy level. The body weight also increased. The radiological investigation suggested that there was a significant improvement in the condition of the patient. Previous studies performed for the treatment of MS using other stem cells have shown good results. Yamout et al., 2010 conducted a pilot scale study on ten patients in which they introduced the mesenchymal stem cells (MSCs) in the MS patients. There was remarkable shift in the EDSS which showed improvement clinically but not radiologically [8]. Previous studies have suggested that the MSC have the homing capability because of their therapeutic effect at the injured or inflamed tissue. The role of chemokines, growth factors, cytokines, transforming growth factors (TGF)-β1, and tumor necrosis factors (TNF)-α regulated the migration of MSC to the injured tissue. They induce the up regulation of selectins and activate the integrins present on the stem cells [9]. Danielyan et al., 2011 reported the migration of MSCs to the olfactory bulb, cortex, hippocampus, striatum, cerebellum, brainstem, and spinal cord through intranasal application in rats with Parkinson's disease. This study showed that in 4.5 months 24% of the cells survived in the brain of the rats resulting in substantial improvement of motor function and decreased concentrations of inflammatory cytokines in the rats [10]. We assume that hESCs used in our study also had the similar mechanism of action. We have previously reported an improvement in our patients with cortical visual impairment wherein we administered hESCs through eye drops and nasal route coupled with the IV and IM routes for faster action [11]. Due to their small size (0.5–2 µm), these cells are able to penetrate the blood brain barrier, reach the brain and repair and regenerate the injured tissues [6]. This is also reflected in the MRI tractography findings of the patient done after the hESC therapy. | ||||||
|
Conclusion
| ||||||
|
Summarizing, we observed a significant improvement in symptoms of a patient with multiple sclerosis, treated with human embryonic stem cells (hESC) therapy in a short duration of time. No adverse event was reported with hESC therapy. Future large scale studies are needed for a better perspective of hESC therapy in patients with multiple sclerosis. | ||||||
|
Acknowledgements
| ||||||
|
I acknowledge Knowledge Isotopes Pvt. Ltd. for their writing support (http://www.knowledgeisotopes. com). | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Geeta Shroff – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2015 Geeta Shroff. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|